For a decade, scientists have studied the application of visible blue light for disinfection.
By 2016, blue light disinfection was in its commercial infancy. Although it’s disinfection properties could be validated in laboratory studies, blue light devices had functional problems. First, the wavelengths that blue light disinfects at needed to be precisely spiked, which early technology could not do well. Second, the dose strength of the blue light was not high enough beyond a couple of inches and not at ceiling height, which blue light disinfection would need to become highly adopted.
As blue light disinfection technology improved, it found immediate usage in healthcare procedures, especially in wound disinfection and dermatology, but it has not yet been proven effective against most viruses because viral strains lacked the phot-catalyzing properies for oxidation and microbial inactivation (it has been proven in some cases today).
Ultraviolet Light Takes Over
Due to the early problems with visible light disinfection, ultraviolet surface disinfection became mainstream – with the introduction of the “UV robot,” a Dr. Who Dalek-looking device that would be placed into hospital rooms to zap surfaces with high-energy ultraviolet UV-C radiation. Today, the Daleks are joined by an army of machines that essentially use the same UV mechanisms – radiating powerful UV energies in unoccupied rooms.
The use of UV light wavelengths for disinfection dates back to the late 19th century, when sunlight was shown to disinfect bacteria in liquid media. Later, in the 1930s, Harvard scientists William F. Wells and Richard L Riley published “Air Contagion and Air Hygiene” and “Airborne Infection: Transmission and Control.” These studies led to the rapid and widespread evolution of UV-C based devices and mechanisms for air disinfection.
Many of these devices were labeled “UVGI”, or ultraviolet germicidal irradiation. Wells first used a UVGI device against measles in a Philadelphia classroom in the 1930s. UVGI was also deployed to combat tuberculosis transmission in the 1950s.
Today, versions of UVGI devices for air disinfection are in use worldwide. But their harmful UV-C irradiance limits their overall effectiveness in occupied spaces because humans can’t be directly exposed. As such, they are banished to HVAC ducts and ceiling devices.
For surface UV-C disinfection, spaces must be unoccupied – then robots can be wheeled in and powered up. While effective, the procedure is a one-time process. UV-C radiation can also degrade surfaces and materials and may induce chemical reactions that create oxidation and/or harmful gas compounds.
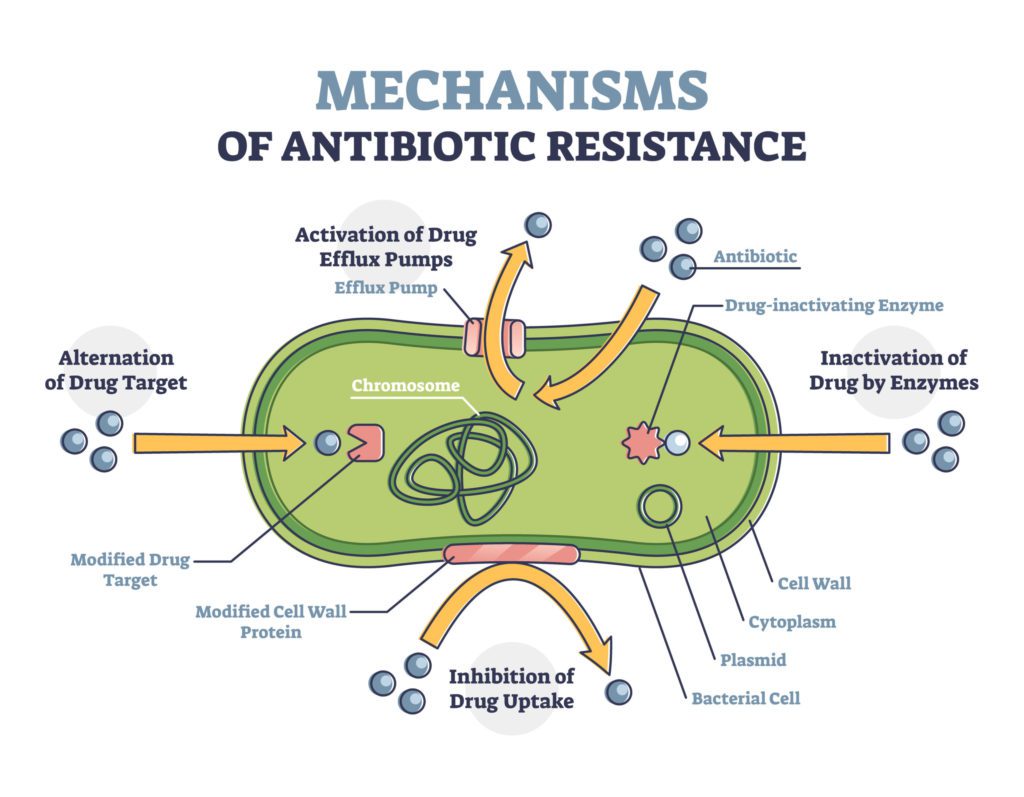
A note of antibiotic-resistant pathogens
Scientists have long been concerned with the growing number of antibiotic-resistant pathogens, including dangerous vegetative bacteria and spore-forming species. Yet, generally, these dangers were not on the world’s collective conscience.
At the same time, hospital-acquired infection (HAI) rates became so alarming that Medicare (CMS) began penalizing hospitals for HAI rates above certain thresholds. Epidemiologists and infectious disease experts understood that ambient air throughout hospitals contained harmful microbes that could enter surgical sites, wounds, and lungs of ventilated patients.
A recent New York Times article expanded on the growing crisis:
“Another possible contributor has been the heavy and regular use of steroids to treat Covid-19. These drugs help alleviate the virus’s most dangerous symptoms but can leave the immune system compromised in a way that allows other germs to more easily infiltrate the body.
The combination of these factors “is perfect” for the fungus to “take hold,” said Dr. Tom Chiller, the head of the fungal division of the C.D.C.
Earlier this month, the Florida Department of Health published a case report of four Candida Auris cases at a hospital in Florida. (The hospital’s identity is masked by the C.D.C. and the state.) In an effort to understand the spread, the Florida department of health visited the Covid unit there in August.
Their inquiry found that 35 of 67 patients admitted to the unit from Aug. 4 to 18 were colonized with C. Auris, meaning that the fungus was on their skin, but they were not yet infected.
Subsequently, six of the patients became infected.
Crucially, the study found that the spread of the fungus from one patient to the next may well have come from health care providers carrying the germ on protective gowns or gloves, as well as on mobile computers and other equipment that had been insufficiently cleaned. This was, the C.D.C. and other experts said, a breakdown in so-called infection control, a practice that had come under intense scrutiny in 2019 after C. Auris took root in the East Coast and began to spread.”
The Consequence of Widespread Resistant Bacteria in Nursing Homes
Resistant bacteria thrive in long-term care facilities and nursing homes. A recent study found that up to 65% of patients in nursing homes have some form of drug-resistant infection.
From the NYT article:
““They are caldrons that are constantly seeding and reseeding hospitals with increasingly dangerous bacteria,” said Betsy McCaughey, a former lieutenant governor of New York who leads the nonprofit Committee to Reduce Infection Deaths. “You’ll never protect hospital patients until the nursing homes are forced to clean up.””
“The story is far bigger than one nursing home or one germ. Drug-resistant germs of all types thrive in such settings where severely ill and ventilated patients like Ms. Davila are prone to infection and often take multiple antibiotics, which can spur drug resistance. Resistant germs can then move from bed to bed, or from patient to family or staff, and then to hospitals and the public because of lax hygiene and poor staffing.”
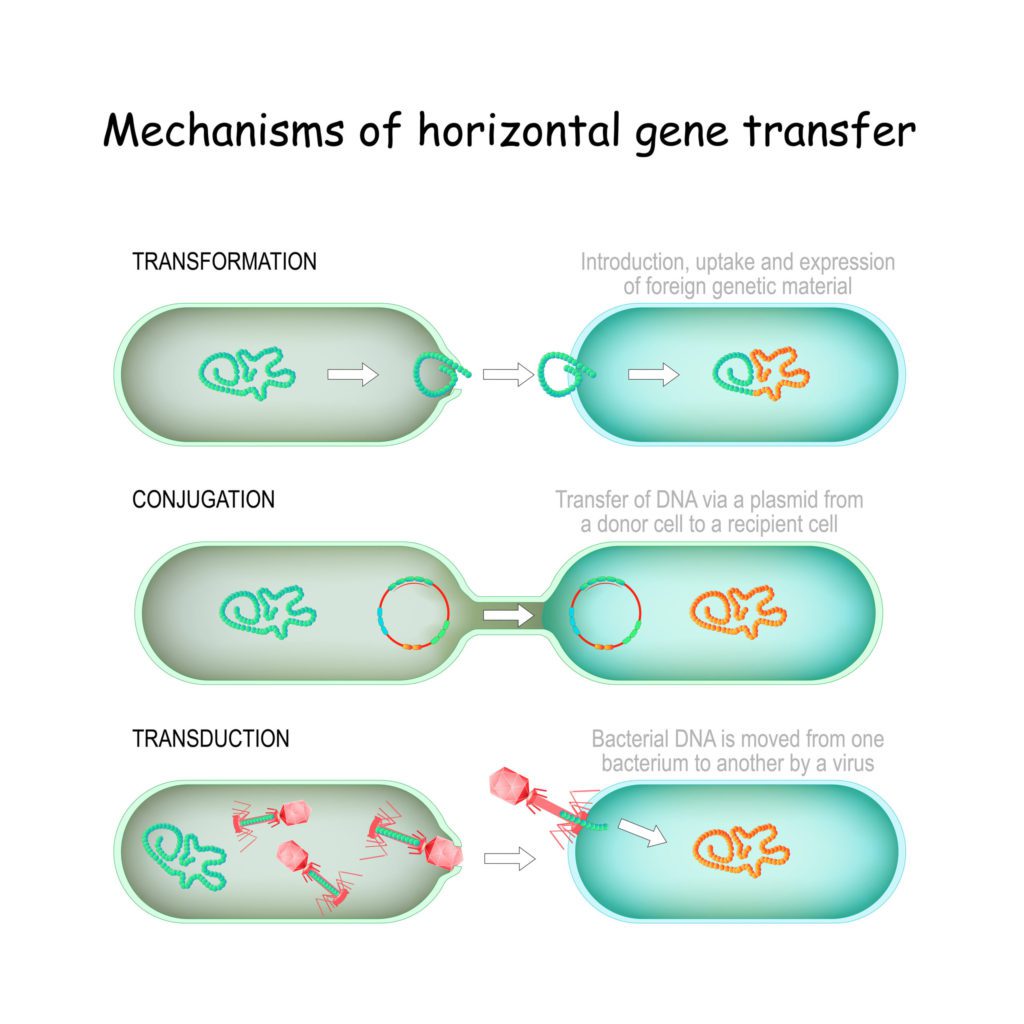
The "Horizontal Transfer" of Resistant Genes between Bacteria
Scientists have learned that bacteria can share their resistance, through a process called “horizontal gene transfer.”
From a study published in ELife, Science Daily wrote:
“Their results determined that many of the mobile elements found in the study were likely being shared among hospital bacteria. In one case, the team identified a plasmid — a circular piece of DNA found in bacterial cells — that encoded multidrug resistance and appeared to have been horizontally transferred between bacteria infecting two separate patients.”
That means that resistant colonies of microbes can grow by transferring antibiotic resistance to one another. Microbes can also develop resistance to certain chemical disinfectants:
“The coats(s) and, to some extent, the cortex in spores, the arabinogalactan and possibly other components of the mycobacterial cell wall and the outer membrane of Gram-negative bacteria limit the concentration of active biocide that can reach the target site(s) in these bacterial cells.
A special situation is found with bacteria present in biofilms, which can be considered as being an intrinsic resistance mechanism resulting from physiological (phenotypic) adaptation of cells.
Acquired resistance to biocides may arise by cellular mutation or by the acquisition of genetic elements. Plasmid/transposon-mediated resistance to inorganic and organic mercury compounds by hydrolases and reductases has been extensively studied.”
COVID-19 and Resistant Pathogens
Infectious disease transmission became a household topic in 2019 with COVID. Within months, the world was introduced to the concepts of disease transmission, surface disinfection, airborne transmission, personal safety, air quality, distancing, and even mRNA vaccines.
As healthcare providers treated millions of COVID-19 cases, the use of antibiotics became essential, as potentially fatal respiratory infections were common in ventilated patients.
Antibiotic-Resistant Pathogens and Light-Based Disinfection
We’ve taken the time to explain antibiotic-resistance because it lays the groundwork for why lighted based disinfection is so important. As can be seen from the above paragraphs, the more bacteria we can eliminate without using antibiotics, the longer antibiotics will remain effective.
As a last line of defense, the continuous illumination of precision, pathogen-reducing visible-light wavelengths has never been more important.
Although the use of UV-C radiation, in both upper-room air and on surfaces (using the “robot” devices in unoccupied rooms) are important tools in the fight against disease transmission, the continuous disinfection of surfaces with blue-light wavelengths is gaining renewed interest.
With constant (or long dose) illumination, colonies of pathogens, including bacterial, spores, mold, and fungus, are reduced, eventually destroyed, and then continuously suppressed. All illuminated surfaces, including clothing, linens, instruments, devices, etc. are bathed in direct or reflected light especially engineered to kill pathogens – even the ones that have become resistant to antibiotics – and perhaps some disinfectants.
Blue-light disinfection therapy is even used now to directly treat infections in vivo, in wounds, surgical sites, and human tissue. Experiments are being conducted on craniotomy sites in mice to determine if 405nm light can disinfect open brain sites where antibiotics are ineffective.
For example, the CleanWhite™ LED fixture emits sharp spikes of 405-nm and 470-nm wavelengths (which were added as ongoing studies showed enhanced efficacy for certain pathogens) – two specific, safe wavelengths known to energize an oxidative process, which is described in a study by Ramakrishnan et al in 2016.
Continuous Disinfection with White-illuminating 405/470 Light
Thus, it is finally time for blue-light disinfection to become a mainstream part of the overall disinfection and infection prevention strategy.
The use cases are virtually endless – schools, hospital settings, clinics, dental practices, restaurants, food production, grow facilities, transportation, ambulance-EMT, fire and police, correctional, dialysis, long term care, nursing homes, athletic departments, hospitality, travel, retail, office space, and many others.
New controls built into the lights (as an optional feature) can sense when rooms or spaces are unoccupied – and can dramatically increase the intensity of the disinfecting wavelengths to shorten kill times. Research has shown that even these higher intensity wavelengths are safe – they just emit light at a higher lumen level.
While air disinfection remains critically important in reducing airborne disease transmission and infection, surface disinfection – every surface – cannot be ignored.
Blue-light disinfection is back. And more important than ever.
Footnotes
[1] Maclean, M., S. J. MacGregor, J. G. Anderson and G. A. Woolsey (2008a) The role of oxygen in the visible-light inactivation of Staphylococcus aureus. J. Photochem. Photobiology B Biol. 92(3), 180– 184.
[2] Maclean, M., S. J. MacGregor, J. G. Anderson and G. Woolsey (2009) Inactivation of bacterial pathogens following exposure to light from a 405-nanometer light-emitting diode array. Appl. Envi- ron. Microbiol. 75(7), 1932–1937.
[3] https://onlinelibrary.wiley.com/doi/abs/10.1111/j.1600-0781.2009.00474.x
[4] Wells WF. Airborne contagion and air hygiene: an ecological study of droplet infections. Cambridge (MA): Harvard University Press; 1955. [Google Scholar] / Riley RL, O’Grady F. Airborne infection: transmission and control. New York: The Macmillan Company; 1961. [Google Scholar]
[5] Cohen CC, Liu J, Cohen B, Larson EL, Glied S. Financial Incentives to Reduce Hospital-Acquired Infections Under Alternative Payment Arrangements. Infect Control Hosp Epidemiol. 2018;39(5):509-515. doi:10.1017/ice.2018.18
[6] https://www.nytimes.com/2021/01/27/health/covid-drug-resistant-infections.html
[7] https://www.nytimes.com/2019/09/11/health/nursing-homes-fungus.html
[8] Daniel R Evans, Marissa P Griffith, Alexander J Sundermann, Kathleen A Shutt, Melissa I Saul, Mustapha M Mustapha, Jane W Marsh, Vaughn S Cooper, Lee H Harrison, Daria Van Tyne. Systematic detection of horizontal gene transfer across genera among multidrug-resistant bacteria in a single hospital. eLife, 2020; 9 DOI: 10.7554/eLife.53886
[9] https://www.sciencedaily.com/releases/2020/04/200414122821.htm
[10] Russell AD. Bacterial resistance to disinfectants: present knowledge and future problems. J Hosp Infect. 1999 Dec;43 Suppl:S57-68. doi: 10.1016/s0195-6701(99)90066-x. PMID: 10658759.
[11] Thurman CE, Muthuswamy A, Klinger MM, Roble GS. Safety Evaluation of a 405-nm LED Device for Direct Antimicrobial Treatment of the Murine Brain. Comp Med. 2019;69(4):283-290. doi:10.30802/AALAS-CM-18-000126
[12] Cytotoxic responses to 405nm light exposure in mammalian and bacterial cells: Involvement of reactive oxygen species DOI link: https://doi.org/10.1016/J.TIV.2016.02.011 Published: 2016-06 [13] See R.M. Tomb et al., “New Proof-of-Concept in Viral Inactivation: Virucidal Efficacy of 405 nm Light Against Feline Calicivirus as a Model for Norovirus Decontamination,” Food & Environmental Virology, Vol. 9(2), pp. 159-67 (2017).